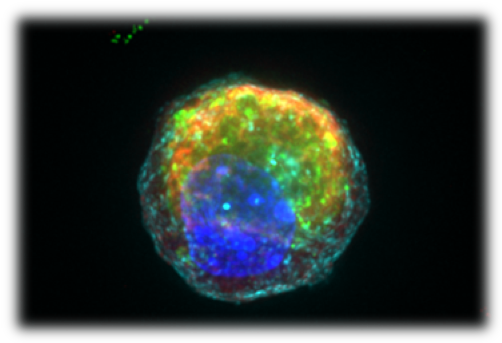
*Photo: Image of a single Paneth cell with anti-microbial granules in red and green, derived from and adult intestinal stem cell, following the small molecule bioengineering approach identified by Mead et al.
For many years, drug development has relied on simplified and scalable cell culture models to find and test new drugs for a wide variety of diseases. However, cells grown in a dish are often a feint representation of healthy and diseased cell types in vivo. This limitation has serious consequences – many potential medicines that originally appear promising in cell cultures often fail to work when tested in patients, and targets may be completely missed if they do not appear in a dish.
A highly collaborative team of researchers from the Harvard-MIT Program in Health Sciences and Technology (HST) and Institute for Medical Engineering and Science (IMES) at MIT recently set out to tackle this issue as it relates to a type of cell found in the intestine that is implicated in inflammatory bowel disease and reported their findings in BMC Biology. The team was led by a student in the HST Medical Engineering and Medical Physics Ph.D. Program, Ben Mead, and a professor at Brigham and Women’s Hospital, Jeffrey Karp, working closely with Jose Ordovas-Montanes, a postdoctoral fellow in the lab of Pfizer-Laubach Career Development Assistant Professor Alex K. Shalek, along with the labs of Professor Jim Collins and Professor Robert Langer and scientists from the Broad Institute of Harvard and MIT and Koch Institute for Integrative Cancer Research.
Understanding genetic risk at the level of single cells
Individual genes can alter one’s risk of developing diseases such as Crohn’s disease, a type of inflammatory bowel disease (IBD). One active area of research is understanding where these genes act in a tissue in order to further our understanding of disease mechanisms and propose novel therapeutic interventions. To address this, techniques are needed to reliably map “risk” genes not only within an affected tissue but to individual cells, to properly surmise if a drug screen can correct a faulty gene or potentially improve a patient’s condition.
Single-cell RNA-sequencing at scale, a revolutionary technique pioneered for low-input clinical biopsies at MIT between Alex K. Shalek’s and Chris Love’s group, now allows researchers to deconstruct a tissue into its elemental components—cells —and identify the key patterns of gene expression which specify each cell type. “Based on the differences between model and actual cell, we utilized a computationally driven bioengineering approach to improve the fidelity of the model,” said Karp. “We believe this approach may be key to unlocking the next generation of therapeutic development from cellular models, including those made from patient-derived stem cells.”
Using single-cell “maps” of models and tissues to re-orient the development of a key cell type
Mapping tissues, such as the small intestine, is highly important in understanding where specific “risk” genes are acting. However, the key advances required to translate findings to the clinic will inevitably be through representative models for the cell types identified as interpreting genes and displaying a disease phenotype. One key IBD-relevant cell type already implicated through genetic studies is known as the Paneth cell, responsible for a key anti-microbial role in the small intestine and defending the stem cell niche.
When adult intestinal stem cells are grown in a dish, they self-organize into remarkable structures known as intestinal organoids: 3D cellular structures that contain many of the cell types found in a real intestine. Nevertheless, how these intestinal organoids correspond to the bona fide cell types found in the intestine has proven challenging for researchers to tackle. To directly address this question, Shalek suggested a “quick” experiment to Mead, which then gave rise to the fruitful collaboration between the labs.
Mead and Ordovas-Montanes developed a single-cell map of the true characteristics of small intestinal cell types as found within the mouse and, when comparing them to what a map of the intestinal-derived organoid looks like, identified several differences, particularly within the key IBD-relevant cell type known as the Paneth cell. Since the field’s map of an organoid didn’t quite correspond to the real tissue, it may have led them astray in the hunt for drug targets.
Fortunately, through their single-cell data, the team was able to learn how the maps were misaligned, and ‘correct’ the developmental pathways which were missing in the dish. As a result, they were able to generate a Paneth cell that is a substantially better mimic of the real cell and can now function to kill bacteria and support the neighboring stem cells which give rise to them.
Translational opportunities afforded by improved representations of tissues
“With this improved cell in-hand, we are now developing a screening platform that will allow us to target relevant Paneth cell biology,” says Mead, who plans to continue the work he started as a postdoctoral fellow in Shalek’s group.
Their approach for generating physiologically faithful intestinal cell types is a major technological advance that will provide other researchers with a powerful tool to further their understanding of the specialized cell states of the epithelial barrier. “As we begin to understand which cell types specifically express genes that alter risk for IBD, it will be critical to ensure the disease-models provide an accurate representation of that cell type,” says Ordovas-Montanes.
“We want to make better cell models to not only understand basic disease biology but also to fast-track development of therapeutics,” says Mead. “This research will have an impact beyond the intestinal organoid community as organoids are increasingly employed for liver, kidney, lung, and even brain research, and our approach can be generalized for relating and aligning the cell types found in vivo with the models generated from these tissues.”