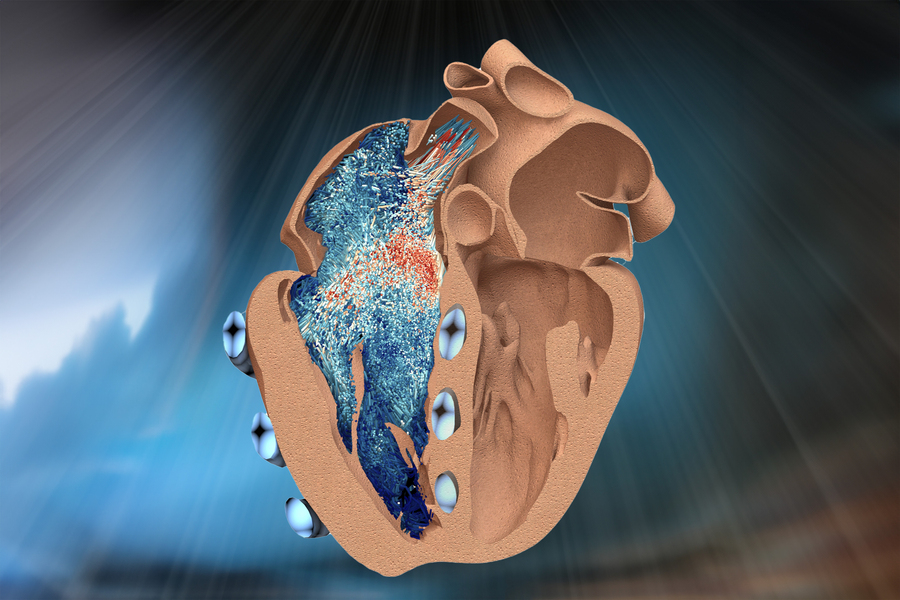
A new bio-robotic model developed by MIT engineers simulates the function of the heart’s lesser-known right ventricle (illustrated here in cross-section, as seen from a front view, on the left). Soft, balloon-like “muscles” (in blue) wrap around and contract the ventricle, mimicking its real pumping action. The model could help to test new implants and devices to treat a range of cardiac disorders.
Credits:
Credit: Courtesy of the researchers
The realistic model could aid the development of better heart implants and shed light on understudied heart disorders.
Jennifer Chu | MIT News
MIT engineers have developed a robotic replica of the heart’s right ventricle, which mimics the beating and blood-pumping action of live hearts.
The robo-ventricle combines real heart tissue with synthetic, balloon-like artificial muscles that enable scientists to control the ventricle’s contractions while observing how its natural valves and other intricate structures function.
The artificial ventricle can be tuned to mimic healthy and diseased states. The team manipulated the model to simulate conditions of right ventricular dysfunction, including pulmonary hypertension and myocardial infarction. They also used the model to test cardiac devices. For instance, the team implanted a mechanical valve to repair a natural malfunctioning valve, then observed how the ventricle’s pumping changed in response.
They say the new robotic right ventricle, or RRV, can be used as a realistic platform to study right ventricle disorders and test devices and therapies aimed at treating those disorders.
“The right ventricle is particularly susceptible to dysfunction in intensive care unit settings, especially in patients on mechanical ventilation,” says Manisha Singh, a postdoc at MIT’s Institute for Medical Engineering and Science (IMES). IMES is HST's home at MIT. “The RRV simulator can be used in the future to study the effects of mechanical ventilation on the right ventricle and to develop strategies to prevent right heart failure in these vulnerable patients.”
Singh and her colleagues report details of the new design in an open-access paper appearing today in Nature Cardiovascular Research. Her co-authors include Associate Professor Ellen Roche, who is an HST faculty member, a core member of IMES, and the associate head for research in the Department of Mechanical Engineering at MIT; along with Jean Bonnemain, Caglar Ozturk, Clara Park, Diego Quevedo-Moreno, Meagan Rowlett, and Yiling Fan of MIT; Brian Ayers of Massachusetts General Hospital; Christopher Nguyen of Cleveland Clinic; and Mossab Saeed of Boston Children’s Hospital.
A ballet of beats
The right ventricle is one of the heart’s four chambers, along with the left ventricle and the left and right atria. Of the four chambers, the left ventricle is the heavy lifter, as its thick, cone-shaped musculature is built for pumping blood through the entire body. The right ventricle, Roche says, is a “ballerina” in comparison, as it handles a lighter though no-less-crucial load.
“The right ventricle pumps deoxygenated blood to the lungs, so it doesn’t have to pump as hard,” Roche notes. “It’s a thinner muscle, with more complex architecture and motion.”
This anatomical complexity has made it difficult for clinicians to accurately observe and assess right ventricle function in patients with heart disease.
“Conventional tools often fail to capture the intricate mechanics and dynamics of the right ventricle, leading to potential misdiagnoses and inadequate treatment strategies,” Singh says.
To improve understanding of the lesser-known chamber and speed the development of cardiac devices to treat its dysfunction, the team designed a realistic, functional model of the right ventricle that both captures its anatomical intricacies and reproduces its pumping function.
The model includes real heart tissue, which the team chose to incorporate because it retains natural structures that are too complex to reproduce synthetically.
“There are thin, tiny chordae and valve leaflets with different material properties that are all moving in concert with the ventricle’s muscle. Trying to cast or print these very delicate structures is quite challenging,” Roche explains.
A heart’s shelf-life
In the new study, the team reports explanting a pig’s right ventricle, which they treated to carefully preserve its internal structures. They then fit a silicone wrapping around it, which acted as a soft, synthetic myocardium, or muscular lining. Within this lining, the team embedded several long, balloon-like tubes, which encircled the real heart tissue, in positions that the team determined through computational modeling to be optimal for reproducing the ventricle’s contractions. The researchers connected each tube to a control system, which they then set to inflate and deflate each tube at rates that mimicked the heart’s real rhythm and motion.
To test its pumping ability, the team infused the model with a liquid similar in viscosity to blood. This particular liquid was also transparent, allowing the engineers to observe with an internal camera how internal valves and structures responded as the ventricle pumped liquid through.
They found that the artificial ventricle’s pumping power and the function of its internal structures were similar to what they previously observed in live, healthy animals, demonstrating that the model can realistically simulate the right ventricle’s action and anatomy. The researchers could also tune the frequency and power of the pumping tubes to mimic various cardiac conditions, such as irregular heartbeats, muscle weakening, and hypertension.
“We’re reanimating the heart, in some sense, and in a way that we can study and potentially treat its dysfunction,” Roche says.
To show that the artificial ventricle can be used to test cardiac devices, the team surgically implanted ring-like medical devices of various sizes to repair the chamber’s tricuspid valve — a leafy, one-way valve that lets blood into the right ventricle. When this valve is leaky, or physically compromised, it can cause right heart failure or atrial fibrillation, and leads to symptoms such as reduced exercise capacity, swelling of the legs and abdomen, and liver enlargement.
The researchers surgically manipulated the robo-ventricle’s valve to simulate this condition, then either replaced it by implanting a mechanical valve or repaired it using ring-like devices of different sizes. They observed which device improved the ventricle’s fluid flow as it continued to pump.
“With its ability to accurately replicate tricuspid valve dysfunction, the RRV serves as an ideal training ground for surgeons and interventional cardiologists,” Singh says. “They can practice new surgical techniques for repairing or replacing the tricuspid valve on our model before performing them on actual patients.”
Currently, the RRV can simulate realistic function over a few months. The team is working to extend that performance and enable the model to run continuously for longer stretches. They are also working with designers of implantable devices to test their prototypes on the artificial ventricle and possibly speed their path to patients. And looking far in the future, Roche plans to pair the RRV with a similar artificial, functional model of the left ventricle, which the group is currently fine-tuning.
“We envision pairing this with the left ventricle to make a fully tunable, artificial heart, that could potentially function in people,” Roche says. “We’re quite a while off, but that’s the overarching vision.”
This research was supported, in part, by the National Science Foundation.
* Originally published in MIT News.